NOW ENROLLING The HARBOR study
Help us learn more about the investigational drug elenestinib for indolent systemic mastocytosis and smoldering systemic mastocytosis.
SEE IF YOU QUALIFYHelp us learn more about the investigational drug elenestinib for indolent systemic mastocytosis and smoldering systemic mastocytosis.
SEE IF YOU QUALIFYIndolent systemic mastocytosis is a rare disease that occurs when abnormal mast cells build up in the body. Mast cells are a type of white blood cell and are a normal part of the immune system. Mast cells help fight infections and are also involved in allergic reactions. When mast cells are activated by a trigger, such as germ or virus, certain foods, medications, heat, and physical or emotional stress, they release histamine and other chemical messengers. In those who have ISM, there are too many mast cells and they release excessive amounts of chemical messengers in the body, resulting in a wide range of symptoms.1,2 The disease features of SSM are a lot like ISM, but in SSM, more mast cells build up in the body.
The HARBOR study is a phase 2/3 trial that is exploring whether elenestinib could be safe and effective in treating ISM and SSM.
References
be 18 years of age or older
be diagnosed with indolent systemic mastocytosis (ISM) or smoldering systemic mastocytosis (SSM) by a healthcare provider
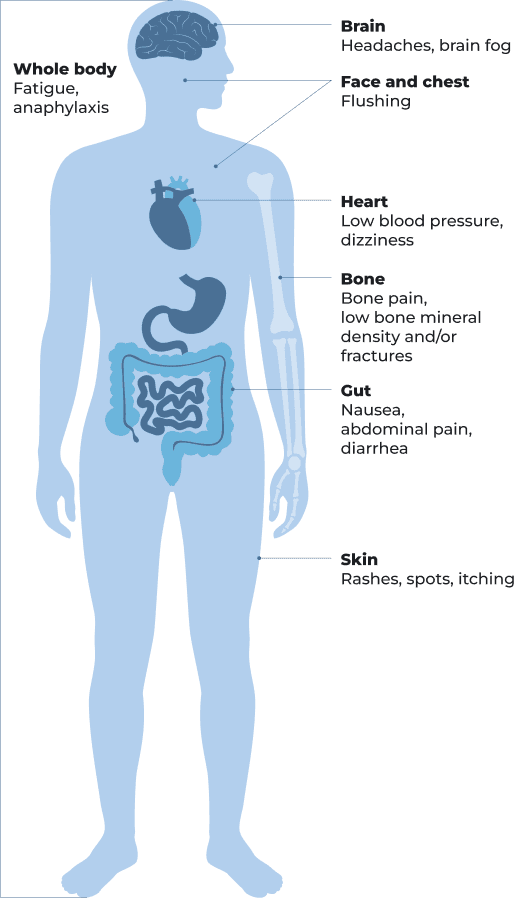
be significantly impacted by symptoms of ISM or SSM (such as abdominal pain, brain fog, bone pain, diarrhea, dizziness, fatigue, flushing, headaches, itching, nausea, and skin rashes) and/or have recurrent anaphylaxis and/or low bone mineral density
be able to travel to a study site to undergo study assessments
To learn more about who can join the HARBOR study and to get more information about participating, please reach out to a study site team near you.
FIND A STUDY SITE NEAR YOUPeople who have indolent systemic mastocytosis (ISM) or smoldering systemic mastocytosis (SSM) suffer from a variety of symptoms, such as skin rashes, itching, flushing, abdominal pain, diarrhea, fatigue, and sometimes even life-threatening allergic reactions (anaphylaxis). Many people try different treatments that target these symptoms, such as antihistamines or other medications, in the hope of feeling better. However, these symptom-directed therapies often do not provide enough relief, and people may continue to experience debilitating symptoms that significantly affect their daily lives. Systemic mastocytosis occurs when abnormal mast cells build up in the body. Mast cells express a receptor called KIT. In approximately 95% of cases, ISM is thought to be caused by a gene mutation or a change in the KIT receptor. The gene mutation is called KIT D816V.
There is a need for additional ISM and SSM treatment options that
Before new medications can be approved for public use, they must be studied in clinical trials. The purpose of a clinical trial is to gather information about an investigational drug and learn whether it works and is safe.
To do this important research, we need volunteers to participate in clinical trials. By participating in the HARBOR study, you will help to increase our understanding of ISM and SSM and find additional ways to treat these conditions in the future.
The HARBOR study is evaluating the safety and efficacy of an investigational drug, called elenestinib, for the treatment of indolent systemic mastocytosis (ISM) and smoldering systemic mastocytosis.
The study will test the study drug against placebo. A placebo pill looks like the study drug, but it has no active ingredients. In addition to the study drug or placebo, participants will continue to receive medication (that they were taking before starting the study) for symptom management.
Study participants will be randomly assigned to receive either the study drug or placebo. Neither the participants nor the study doctor will know which medication the participants will be taking. This is called a double-blinded study. The double-blind period of the HARBOR study lasts 48 weeks.
Participants who complete the 48 weeks of treatment will have the option to receive the active study drug during an open-label extension period (when all participants receive the study drug, regardless of whether they were initially assigned study drug or placebo). The open-label extension period will continue for approximately 4 years. The total participation time for each person in the HARBOR study is approximately 5 years.
In this study, researchers want to learn
The HARBOR study will also include two exploratory groups that will enable the researchers to learn more about the effects of the study drug in participants who have
Indolent systemic mastocytosis, or ISM, is a rare disease that occurs when abnormal mast cells build up in the body. Mast cells express a receptor or “switch” called KIT on the surface that controls the growth, survival, and activation of cells.
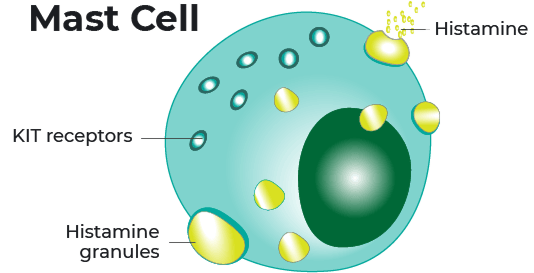
In approximately 95% of cases, ISM is thought to be caused by a gene mutation or a change in the KIT receptor. The gene mutation is called KIT D816V.
Elenestinib is an investigational drug designed to target and inhibit, or block, the KIT D816V mutation. By targeting this mutation, elenestinib aims to reduce the abnormal growth and accumulation of mast cells.
Elenestinib is being developed to potentially
Elenestinib is considered investigational because it is not yet approved by the U.S. Food and Drug Administration (or any other health authority) for the treatment of any disease or condition.
Indolent systemic mastocytosis is the most common type of systemic mastocytosis and accounts for 70% to 80% of cases.1 Indolent systemic mastocytosis typically does not affect organ function. The symptoms may develop over time and can have a significantly negative impact on quality of life.
Reference